1. To observe a variety of chemical reactions.
2. To interpret and explain observations with balanced chemical equations.
3. To classify each reaction as one of the four main types (Synthesis, Decomposition, Double Replacement, and Single Replacement).
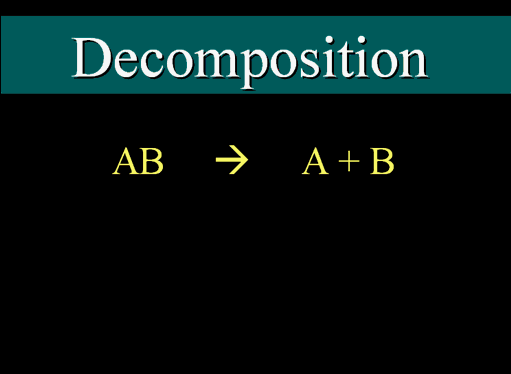 |
| Decomposition |
| Synthesis |
| Single Replacement |
 |
| Double Replacement |
- Equipment: lab burner, 6 test tubes (one will be flame heated), test tube clamp, medicine dropper, wood splints, crucible tongs, steel wool, safety goggles, lab aprons.
- Chemical Reagents: copper wire (bare), iron nail, 0.5M sodium carbonate solution, mossy zinc, 2M hydrochloric acid solution (6%), manganese (IV) oxide.
Procedure
1. Lab aprons + safety goggles.
2. Observations before, during and after each reaction. Record.
Reaction 1
1. Burner (high heat).
2. Hold copper in the hottest part for a few minutes (crucible tongs).
Reaction 2
1. Clean iron nail till shiny with steel wool.
2. Tube with CuSO4, half of nail covered.
3. After 15 min, remove nail, note changes of solution and nail.
Reaction 3
1. 1/3 of tube filled with solid Copper (II) sulphate pentahydrate.
2. Heat the tube, move gently over flame.
3. Heat till no changes observed.
4. Save content for Reaction 4.
Reaction 4
1. Wait till the tube from 3 cool.
2. Medicine dropper: 2 to 3 drops of water.
Reaction 5
1. Test tube 1: 1/4 full of CaCl2 solution, test tube 2: 1/4 full of Na2CO3 (aq).
2. Pour the content in test tube 1 into 2.
Reaction 6
1. Test tube: mossy zinc
2. Add HCl (aq) till zinc is fully covered by HCl
Reaction 7
1. Test tube: 1/2 hydrogen peroxide (aq)
2. Small amount of MnO2 (catalyst)
3. Put a glowing but not burning splint into mouth of the tube to test the gas.
4. Wash hands thoroughly before leaving.
Place all liquid and solid waste into the designated waste containers.
Observations
Reaction 1
Before: copper: orange
During: the flame turns from blue to green almost instantly; copper gradually turns black.
After: copper: black
Reaction 2
Before: nail: silver; CuSO4 (aq): sky blue
During: nail covered by CuSO4 gradually turns orange; CuSO4 turns from blue to dark green.
After: nail: thicker with orange powder-like copper sticking onto the nail; liquid: dark green
Reaction 3
Before: CuSO4·5H2O: sky blue solid in powder form.
During: CuSO4·5H2O gradually turns from blue to white, from outside to inside, water drops form at the mouth of the test tube; there was a little white steam; some white powder gradually turns black or even orange/yellowish in the end.
After: CuSO4: white, partially yellow/orange-ish solid (one big piece, no more powder)
Reaction 4
Before: CuSO4: white, orange-ish solid (one big piece)
During: instantly turns blue
After: CuSO4·5H2O: sky blue solid (one big piece)
Reaction 5
Before: Na2CO3: clear liquid; CaCl2: clear liquid.
During: instantly forms white solid and clear liquid.
After: white solid (CaCO3) in transparent liquid (NaCl)
Reaction 6
Before: zinc: silver metal; HCl: clear liquid
During: instantly starts to form small bubbles; the amount of zinc lessens.
After: clear liquid (ZnCl2) and a little mossy zinc.
Reaction 7
Before: H2O2: clear liquid; MnO2: black powder
During: instantly starts to form a lot of big bubbles (really active); glowing stick starts to burn; test tube gets really hot; white steam
After: black liquid (black powder in clear liquid)
Questions That Might Help You to Understand the Lab Better
1. Q: What types of reactions are reaction 1 to 7?
A: 1 Synthesis, 2 Single Replacement, 3 Decomposition, 4 Synthesis, 5 Double Replacement, 6 Single Replacement, 7 Decomposition
2. Q: Chemical equations for reaction 1 to 7?
A: 1) 2Cu (s) + 1O2 (g) ---> 2CuO (s)
2) 1CuSO4 (aq) + 1Fe (s) ---> 1Cu (s) + 1FeSO4 (aq)
3) 1CuSO4·5H2O (s) ---> 1CuSO4 (s) + 5H2O (l)
4) 1CuSO4 (s) + 5H2O (l) ---> 1CuSO4·5H2O (s)
5) 1Na2CO3 (aq) + 1CaCl2 (aq) ---> 1CaCO3 (s) + 2NaCl (aq)
6) 1Zn (s) + 2HCl (aq) ---> 1ZnCl2 (aq) + H2 (g)
7) 2H2O2 (aq) MnO2 > 2H2O (l) + 1O2 (g)
2. Q: What are some errors students may make during the lab?
A: R1) Burner is not turned to high heat so Cu and O2 do not react.
R2) Iron nail is not cleaned with steel wool; there might be FeO on the surface of the nail; therefore there might be changes in observation.
R7) A burning splint is put into the mouth of the test tube, therefore no obvious changes are noted.
Or, a glowing splint is put at the mouth of the test tube, therefore no obvious changes are noted.
3. Q: What is copper reacting with in reaction 1?
A: Oxygen in the air.
4. Q: What does the colour change in reaction 2 indicate?
A: It indicates that Cu2+ released in water produces the blue colour, Fe2+ replaced Cu2+ to cause the colour change.
5. Q: How to test H2 and O2?
A: H2: place a BURNING stick at the mouth of the test tube. If the gas starts burning or create a little "boom" sound, it's H2.
O2: place a GLOWING stick at the mouth of the test tube. If the stick starts burning, the gas is O2.
6. Q: What do you learn from this lab?
A: There are 4 main different types of reactions, decomposition (with one reactant forming two or more products), synthesis (with two or more reactants forming one product), single replacement (with one single-element reactant and one double-element reactant forming another single-element product and another double-element product) and double replacement (with two reactants switching + ions to get two products).\
7. Q: What types of reactions are not included in this lab?
A: Neutralization (acid + base = salt + water, a special double replacement reaction) and combustion (burning of organic material in oxygen).