Chemical Bonds
Chemical Bonds
- are formed when the electrons of one atom are attracted by the nucleus if another atom.
- are formed with various degrees of sharing of electrons between two atoms
- COVALENT BOND is formed when the electrons are shared equally between two atoms
- When the electrons of one atom are given away completely to another atom, both a positively charged ion and a negatively charged ion are formed
- The force holding the two ions together is called an IONIC BONDS
 |
| Covalent Bond |
 |
| Ionic Bond |
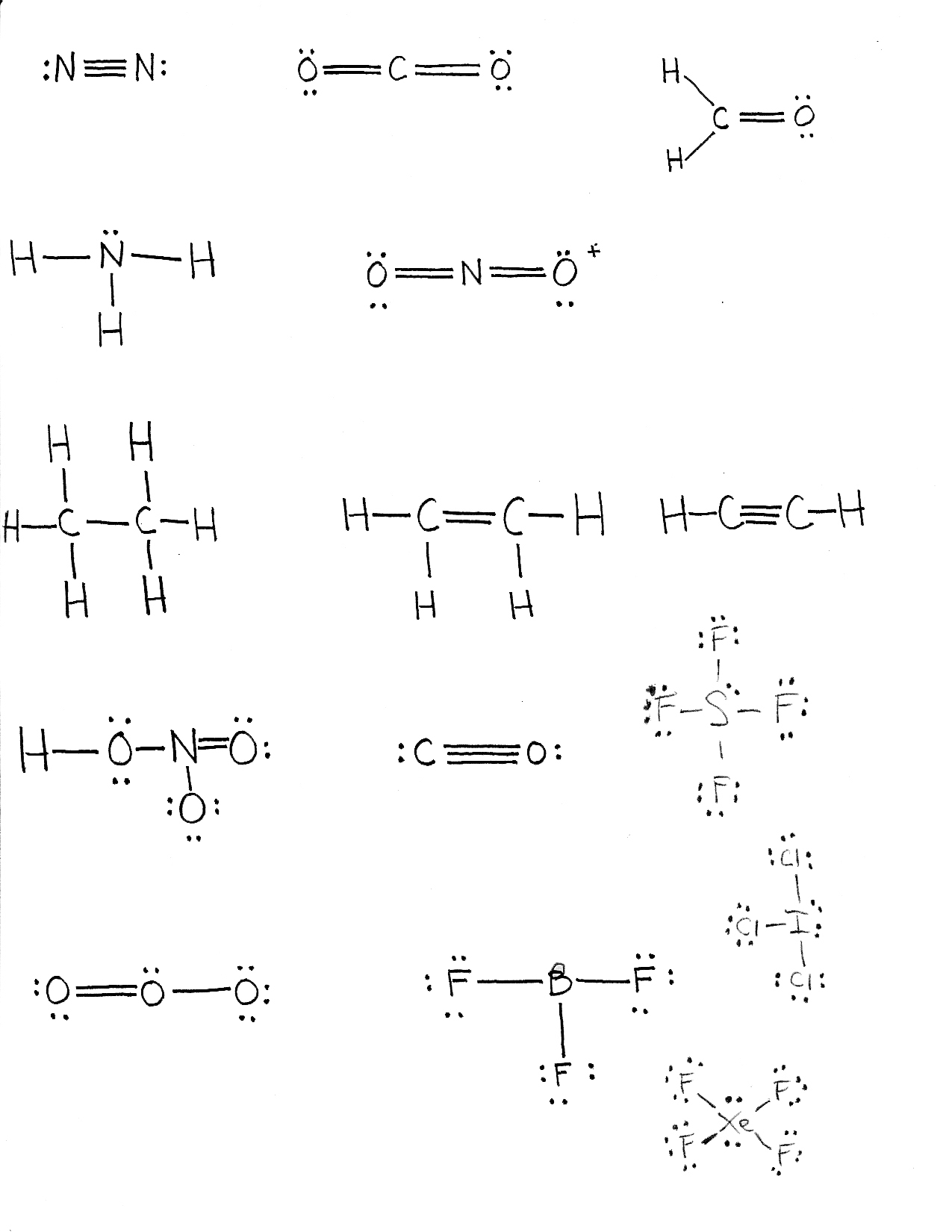
Lewis Structure
- represents valance electrons.
- uses dots to represent electrons, focuses on an atom's valence electrons only.
- The electrons that are in pairs are Lone pairs or Nonbonding electrons.
- These are not used to bond since the orbital they are in already has its full complement if electrons.
- The unpaired electrons are Bonding electrons. They are capable of making one single covalent bond.
- An atom needs to have 2 bonding electrons when we make a double covalent bond.
- To make a triple covalent bond, an atom needs 3 bonding electrons.
 |
| Now single covalent bond is forme |
 |
| Double Bond |
 |
| Triple Bond |
Example of Lewis Structure
No comments:
Post a Comment